Background: Valoctocogene roxaparvovec is expected to transfer a functional FVIII coding sequence that would eliminate the need for regular prophylaxis for people with severe hemophilia A (HA). At the same valoctocogene roxaparvovec dose, FVIII levels varied among participants in the phase 3 GENEr8-1 trial. The protective effect of low transgene-derived FVIII is unknown.
Aim: To determine clinical outcomes for participants with low FVIII activity 3 years post-gene transfer in GENEr8-1.
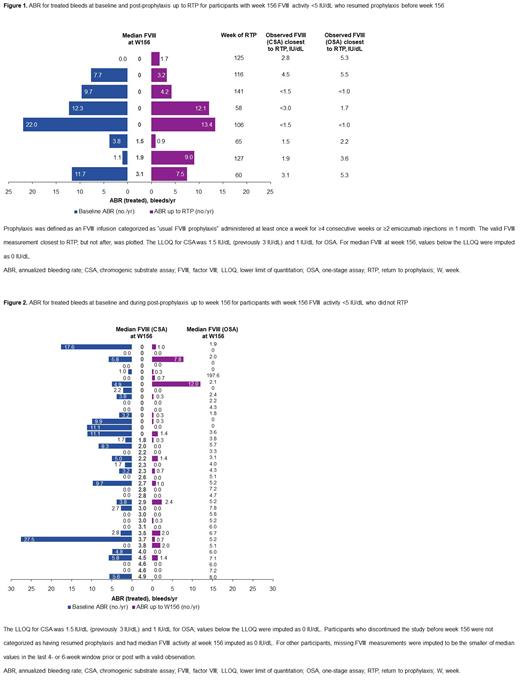
Methods: In the open-label, single-arm, phase 3 GENEr8-1 trial (NCT03370913), 134 adult males (intention-to-treat [ITT] population) with severe HA (FVIII activity <1 IU/dL) previously receiving regular FVIII prophylaxis and with no history of FVIII inhibitors received a single dose of 6x10 13 vg/kg valoctocogene roxaparvovec. Plasma FVIII activity was measured via chromogenic substrate assay (reported in text; lower limit of quantitation [LLOQ], 1.5 [previously 3.0] IU/dL) or one-stage assay (LLOQ, 1.0 IU/dL). For participants who resumed prophylaxis, the valid FVIII measurement closest to, but not after, return to prophylaxis (RTP) is reported. Presented FVIII activity at a visit week is the median value in the 4- or 6-week window around the target date. Week 156 FVIII activity was imputed as 0 for participants who discontinued; other missing FVIII measurements were imputed as the smaller of valid values immediately prior to or post week 156. Bleeds were self-reported during baseline and after cessation of regular FVIII prophylaxis (scheduled for week 4 post-infusion). RTP was defined per-protocol as usual FVIII prophylaxis administered ≥1 time/week for ≥4 consecutive weeks or ≥2 emicizumab injections/month. Outcomes are reported for up to 156 weeks.
Results: Of 134 ITT participants, 131 completed the week 156 visit. Mean (standard deviation [SD]) FVIII activity at week 156 was 18.2 (30.6) IU/dL in the ITT population. At week 156, 46 of 134 (34.3%) ITT participants had median FVIII activity <5 IU/dL (range, 0 to 4.9 IU/dL). These 46 participants had annualized bleeding rate (ABR) for treated spontaneous and traumatic bleeds at baseline between 0 and 27.5 (mean, 4.8) bleeds/year and from post-prophylaxis to week 156 between 0 and 12.0 (mean, 1.7) bleeds/year.
Of these 46 participants, 8 resumed prophylaxis before week 156 (range, 58-141 weeks; Figure 1) and 38 did not RTP before week 156 ( Figure 2). The 8 participants who resumed prophylaxis had FVIII activity between <1.5 and 4.5 IU/dL proximal to RTP; the 38 who did not RTP had median FVIII activity between 0 and 4.9 IU/dL at week 156. At week 156, 3 of 8 and 15 of 38 participants who did and did not RTP had FVIII of 0 IU/dL, respectively.
For the 8 participants who resumed prophylaxis before week 156, baseline ABR for treated bleeds ranged from 0.0 to 22.0 (mean, 8.5) bleeds/year, compared with 0 to 27.5 (mean, 4.0) bleeds/year for the 38 participants who did not RTP before week 156. Treated ABR from post-prophylaxis to RTP ranged from 0.9 to 13.4 (mean, 4.8) bleeds/year for the 8 participants who resumed prophylaxis before week 156, compared with 0 to 12.0 (mean, 1.0) bleeds/year from post-prophylaxis to week 156 for the 38 participants who did not RTP before week 156.
For 2 of 8 participants who resumed prophylaxis before week 156, treated ABR was higher during the post-prophylaxis period up to RTP than at baseline; treated ABR was higher post-prophylaxis to week 156 compared with baseline for 5 of 38 participants who did not RTP before week 156. Most of the 38 participants who did not RTP before week 156 had lower ABR for treated bleeds compared with baseline, low post-prophylaxis ABRs for treated bleeds, or no substantial treated spontaneous bleeds. Decisions to not RTP were multifactorial and based on participant-investigator shared decision making; narratives outlining determinants that may have influenced decisions around prophylaxis for participants who had week 156 FVIII activity <5 IU/dL will be presented.
Conclusions: Most participants with low FVIII activity had low bleeding rates, suggesting that low endogenous FVIII expression may provide protective hemostatic benefits. Many participants who resumed prophylaxis had clinical presentation consistent with moderate hemophilia; the individual decision to RTP was multifactorial and influenced by FVIII activity, bleeding rates, desired physical activity levels, and personal preferences.
Disclosures
Mahlangu:Spark Therapeutics: Research Funding; Novo Nordisk: Research Funding; Roche: Research Funding; Sandoz: Research Funding; Sanofi: Research Funding; Pfizer: Research Funding; BioMarin Pharmaceutical Inc.: Research Funding; Catalyst: Research Funding. Ozelo:BioMarin Pharmaceutical Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Research Funding; Roche: Honoraria, Research Funding, Speakers Bureau; Sanofi: Honoraria, Research Funding; Takeda Development Center Americas, Inc: Honoraria, Research Funding, Speakers Bureau; Novo Nordisk: Honoraria, Research Funding, Speakers Bureau; Biotest: Honoraria; Bayer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Giermasz:Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; BioMarin Pharmaceutical Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants; uniQure: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi Genzyme: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bioverativ, Genentech/Roche, Biomarin, uniQure, American Thrombosis and Hemostasis Network: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Wang:Takeda: Consultancy, Speakers Bureau; Sobi: Consultancy; Sanofi: Consultancy, Other: Clinical trial investigator, Speakers Bureau; Pfizer: Consultancy, Other: Clinical trial investigator, Speakers Bureau; Novo Nordisk: Consultancy, Other: Clinical trial investigator, Speakers Bureau; Chugai: Consultancy, Other: Clinical trial investigator, Speakers Bureau; BioMarin Pharmaceutical Inc.: Other: Clinical trial investigator; Bayer: Consultancy, Other: Clinical trial investigator, Speakers Bureau; CSL: Speakers Bureau; Roche: Other: Clinical trial investigator; Behring: Speakers Bureau. Osmond:BioMarin Pharmaceutical Inc.: Current Employment, Current equity holder in publicly-traded company. Yu:BioMarin Pharmaceutical Inc.: Current Employment, Current equity holder in publicly-traded company. Robinson:BioMarin Pharmaceutical Inc.: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal